Platelets toolkit
This page is for healthcare professionals to ensure platelets are used safely and appropriately.
According to National Comparative Audit reports, platelets are often used inappropriately and outside of guidelines.
As the potential donor population for A D negative platelets is only 7%, they are a valuable resource and should not be used as a ‘universal’ component.
You should regularly review your platelet ordering patterns, activity and wastage. Contact your Customer Service Manager or the Blood Stocks Management Scheme for advice on stocking an alternative to A D negative platelets.
In this section
Pages in the 'Appropriate use of blood components' section
Infographics
These infographics are designed for you to use alongside appropriate text in presentations and educational resources. Please acknowledge the NHS Blood and Transplant Patient Blood Management team as the author.
Establish a strategy for platelets
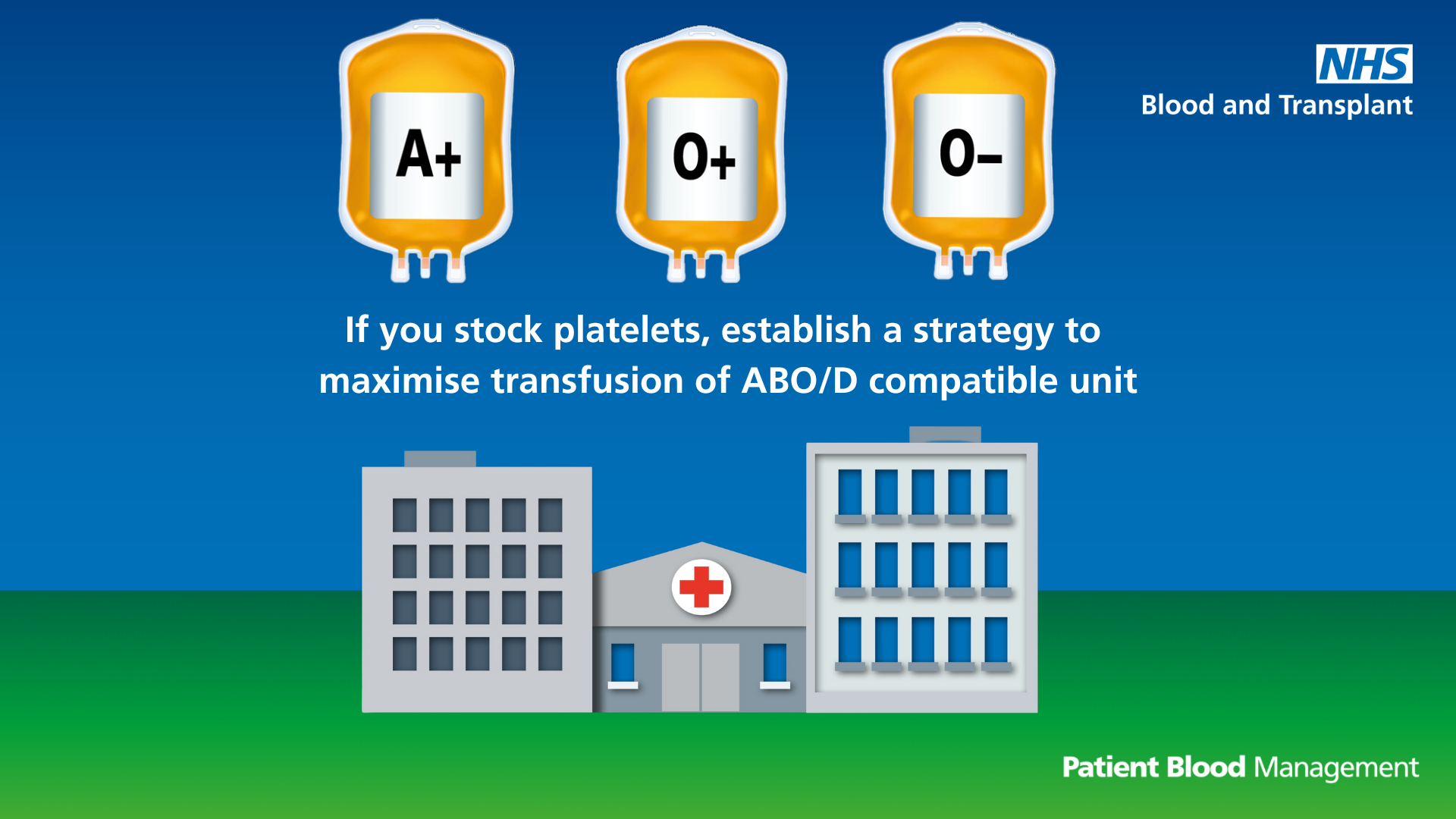
Text reads: "If you stock platelets, establish a strategy to maximise transfusion of ABO/D compatible units."
Download the ABO/D compatible platelets infographic (PDF 746KB)
Limit use of platelets of a different group
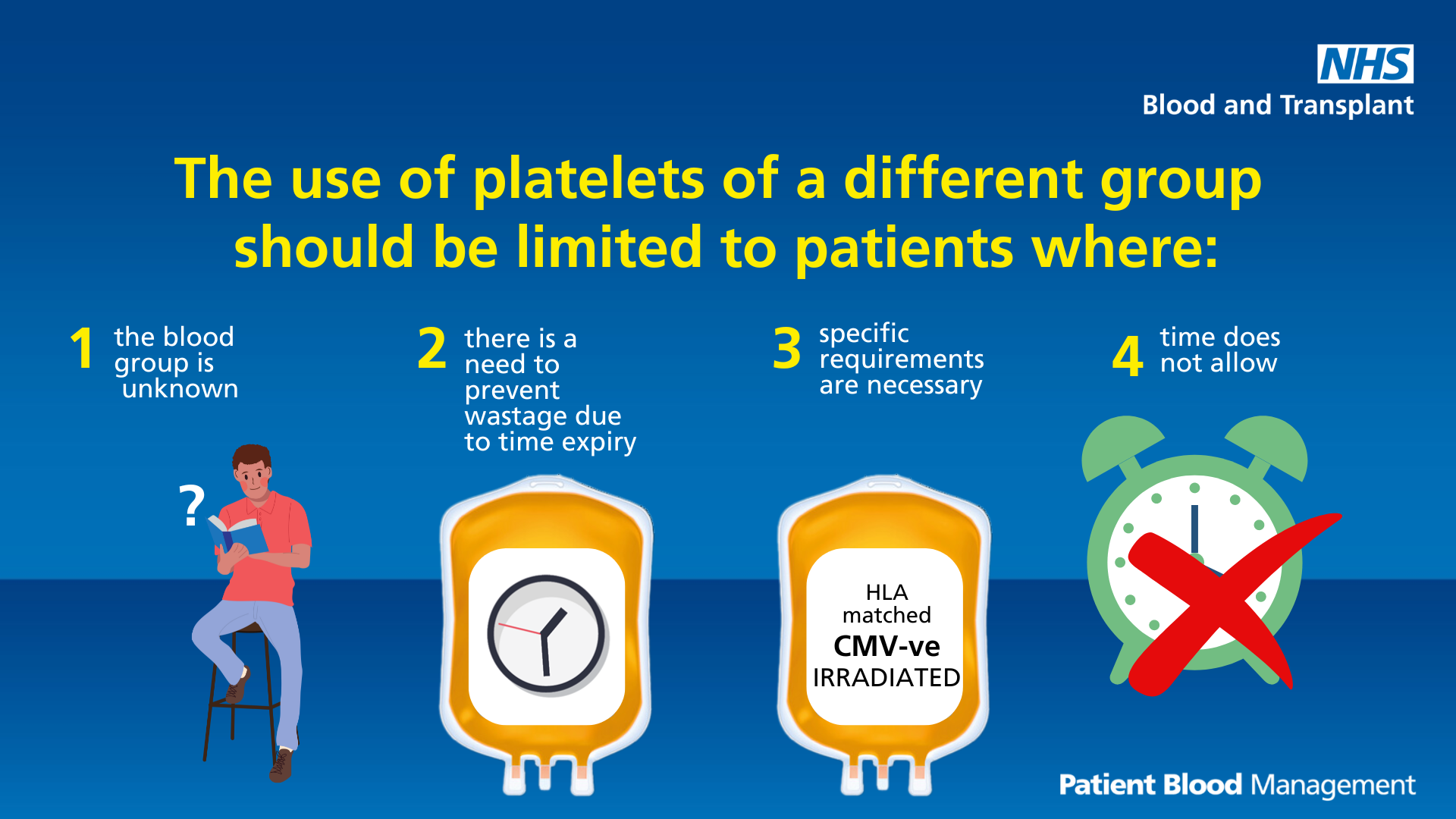
Text reads: "The use of platelets of a different group should be limited to patients where:
- The blood group is unknown
- There is a need to prevent wastage due to time expiry
- Specific requirements are necessary
- Time does not allow"
Download the platelets of a different group infographic (PDF 493KB)
Don’t use two when one will do

Text reads: "Don’t use two when one will do. One adult therapeutic dose of platelets is required for prophylaxis."
Prophylactic platelets
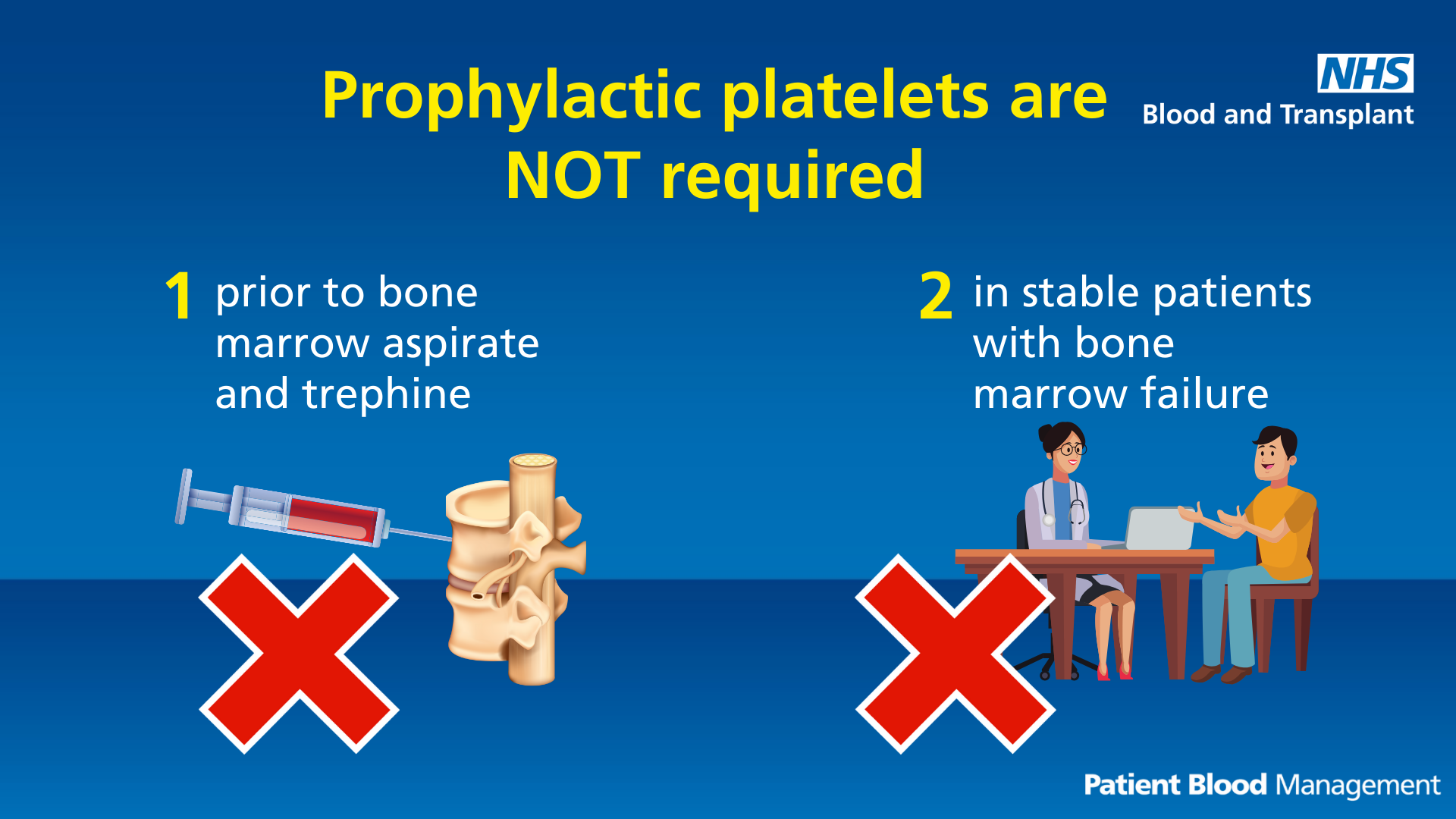
Text reads: "Prophylactic platelets are not required:
- Prior to bone marrow aspirate and trephine
- In stable patients with bone marrow failure"
CMV negative components
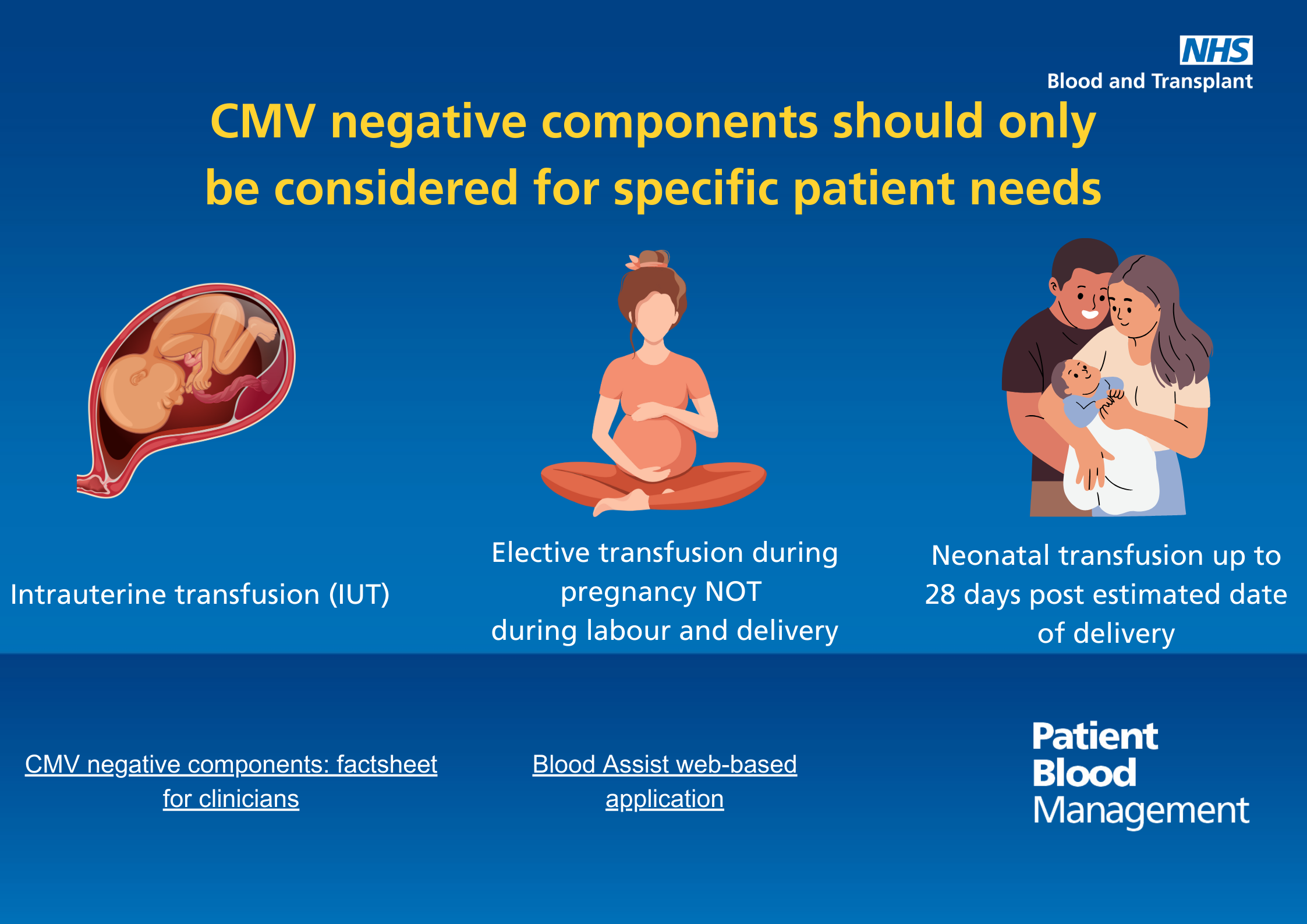
Text reads: "CMV negative components should only be considered for specific patient needs.
- Intrauterine transfusions
- Elective transfusion during pregnancy, not during labour or delivery
- Neonatal transfusions up to 28 days post estimated date of delivery"
Download the CMV negative components infographic (PDF 1,475KB)
Share your learning
Tell us about your projects relating to platelets, highlighting your successes or where things didn't go so well, and we'll include them on this page.
Contact us at: pbm.team@nhsbt.nhs.uk
Page last reviewed: 10 February 2025